It is no surprise that not all drugs are equal. While some drugs have minimal side effects, others have more serious ones, along with risks for adverse events. If there are safety concerns about the use of a drug, the U.S. Food and Drug Administration (FDA) applies warnings. The type of warnings found on prescription drug labeling vary depending on the type of side effects. An FDA black box warning draws attention to the drugs with more serious side effects, such as heart failure. The warning alerts prescribers and patients that the drug could have serious or life-threatening risks.
What is a black box warning?
A black box warning, or “boxed warning,” alerts consumers about serious or life-threatening side effects the drug may have. Found on the approved prescribing information, or drug label package insert for prescription drugs, it’s the most serious warning given by the FDA. The text is bold-faced, surrounded by a black border—giving the warning its name—to call attention to a medication’s risks.
What does a black box warning mean?
A drug gets a black box warning when it has potentially serious adverse reactions that could lead to hospitalization and death or permanent damage. The strongest FDA warning issued, a black box warning also explains how the reactions may be worse in certain groups of people, such as women who are pregnant, pediatric patients, and elderly patients.
All drug companies in the United States must go through testing before FDA approval. During this testing, a drug may have side effects that call for a black box warning before it goes to market. To identify such medications, the FDA relies on clinical trial data as well as the reporting of serious adverse events by clinicians and patients. On occasion, during these clinical trials for new drugs, every side effect may not be uncovered, since testing usually occurs in only a few thousand patients. There could be more serious side effects found later, after the drug is already approved, when it’s used in tens or hundreds of thousands of patients. When this happens, black box warnings may be added to the medicine after it has hit the market. Similarly, a black box warning can be removed if new clinical evidence shows that its risks are less severe.
Where can I find black box warnings?
While black box warnings will appear on the bottle or package insert for a medication, the warning will also appear on the patient information sheet that your pharmacy provides when a prescription is filled.
Which drugs have a black box warning?
“Over 400 medications have black box warnings and 40% of people are taking at least one medication with a black box warning,” says Rick Rayl, R.Ph, the director of pharmacy at the University Behavioral Health of Denton in Texas. Here are some of the most commonly prescribed drugs with black box warnings listed on the medication guide.
| Drug class | Drug name | Reason for warning |
| Antidepressants/Selective Serotonin Reuptake inhibitors (SSRIs) | Zoloft (sertraline hydrochloride)
Celexa (citalopram hydrobromide) |
All antidepressants received a black box warning in 2004 to warn consumers about an increase in suicidal thoughts and behaviors associated with these drugs for people with mental health problems. |
| Bronchodilators/LABAs and steroidal anti-inflammatory agents | Advair Diskus (fluticasone salmeterol) | Increased risk of asthma-associated deaths |
| Miscellaneous antipsychotic agents | Seroquel (quetiapine fumarate) | Increased mortality in the elderly that have
dementia-related psychosis and increase of suicidality in children through young adults |
| Atypical antipsychotic agents | Clozaril (clozapine) | Agranulocytosis, a condition that can lead to severe infection and death |
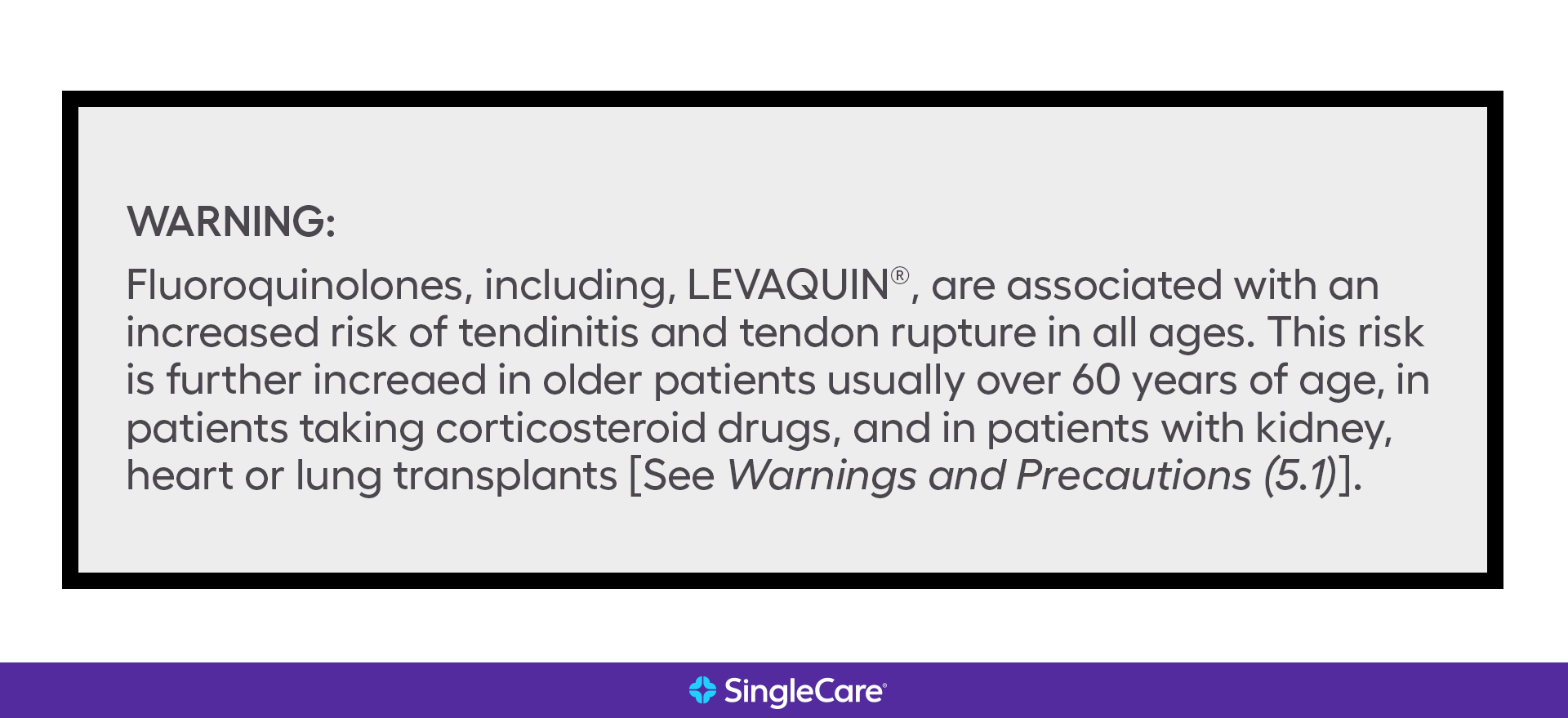
| Antibiotics (fluoroquinolones) | Levaquin (levofloxacin) | Risk of tendinitis and tendon rupture across all ages |
| Antimetabolites or folic acid antagonists | Methotrexate | Increased risk of end-stage liver disease |
| Antiarrhythmic | Pacerone (amiodarone) | Increased risk of lung and liver toxicity and heart problems |
| Antimalarials | Mefloquine | Increased risk of permanent dizziness, tinnitus, and loss of balance |
| Monoclonal antibodies | Tysabri (natalizumab) | Increased risk of progressive multifocal leukoencephalopathy, an often terminal disease of the brain’s white matter |
| Opioids | Oxycontin (oxycodone) | Risk of addiction, abuse, and life-threatening breathing difficulties |
| Combination birth control pills containing drospirenone | Ocella
Gianvi Zarah |
Increased risk of blood clots |
| Thiazolidinediones | Duetact (Pioglitazone /Glimepiride) | Increased risk of bladder cancer |
| Biguanides | Riomet (metformin) | Risk of lactic acidosis |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Motrin, Advil (ibuprofen) | Increased risk of heart attack and stroke; increased risk of gastrointestinal bleeding, ulceration, and holes in the stomach or intestines |
| Benzodiazepines | Xanax (alprazolam) | Risk of misuse, abuse, and addiction |
Benzodiazepines like Xanax and Ativan received a black box warning from the FDA in 2020. That’s because even when taken as directed, these medications can lead to misuse, abuse, and even addiction. What’s more, benzodiazepines can cause physical dependence even at regular doses, resulting in withdrawal symptoms such as life-threatening seizures when the drug is stopped suddenly or dosage is reduced too quickly. Another black box warning was previously in place for benzodiazepines to warn against their use with opiates—when these two drug classes are combined, extreme drowsiness, breathing issues, coma, and even death may result.
See an example of a black box warning:
Issues with black box warnings
Although black box warnings are issued to alert physicians and patients about a medication’s serious or fatal side effects, or potential to cause permanent damage, an observational study of 51 outpatient centers indicated that the warnings sometimes go ignored by prescribers. Even so, these serious adverse events are usually quite rare, as less than 1% of patients who received a drug in violation of a black box warning experienced serious adverse effects as a result of taking the drug.
And while a black box warning warns the public about the risks of a drug, it may discourage people from taking a medication with a potential benefit. For example, one study showed that black box warnings about suicidality risk assigned to certain antidepressant medications caused a persistent decline in the treatment of major depressive disorder, resulting in a significant increase in the rate of suicide attempts.
Though they’re a useful tool to convey the risks of potentially dangerous medications, researchers say that the FDA’s process of adding or removing black box warnings lacks transparency. And drugs whose approval is expedited by the FDA are more likely to have black box warnings added after they’ve already been prescribed to patients.
What to do if your medication has a black box warning
When starting any new prescription, it is important to go over the drug safety information with your doctor or pharmacist. The decision to take a prescription that has an FDA black box warning should not be taken lightly and should be discussed with your healthcare provider. A conversation with healthcare professionals is the best way to determine if the benefit of the drug outweighs the potential risk in these scenarios.
“As with all medications, there is a benefit and a cost to consider,” Rayl says. “Consult your pharmacist with questions that will help you decide if this is the right medicine for you.”